MEDTECH
Empowering health innovation through science, systems, and precision.
MedTech is rapidly changing the way we diagnose, monitor, and treat disease. We partner with innovators working on diagnostics, digital health, and medical devices—bringing clarity to complex systems through scientific rigor, regulatory insight, and smart data solutions.
Our support
MedTech companies that align early with regulatory and clinical expectations see a 20–25% faster time-to-market and significantly higher investor confidence. Our support in experimental design, validation, and compliance helps you bring safe, effective technologies to patients — faster and more efficiently.
Whether you’re developing a diagnostic device, refining a digital health tool, or validating a new medical sensor, we offer the scientific and technical backbone to help you move with confidence. Our team collaborates closely with R&D leads, clinicians, and lab technicians to design effective protocols, structure experiments, and navigate compliance from bench to bedside. With our support, you can reduce trial-and-error, generate stronger data, and accelerate your journey from prototype to product — all while staying aligned with regulatory expectations.

FEATURED CAPABILITIES

GUIDE LAB
Setting up a MedTech lab — whether it’s for diagnostics, wearables, or biomaterials — requires technical insight and regulatory awareness. We guide you in designing lab spaces, selecting compliant equipment, writing SOPs, and aligning with ISO or FDA requirements.
Our service is especially valuable during early development, scale-up, or audit preparation.
AI AND ALGOS
We develop intelligent algorithms for signal processing, imaging, predictive diagnostics, and decision support tools.
Whether you’re analyzing ECGs, detecting anomalies in medical imaging, or personalizing treatment plans, our models are built with clinical relevance and regulatory transparency in mind.
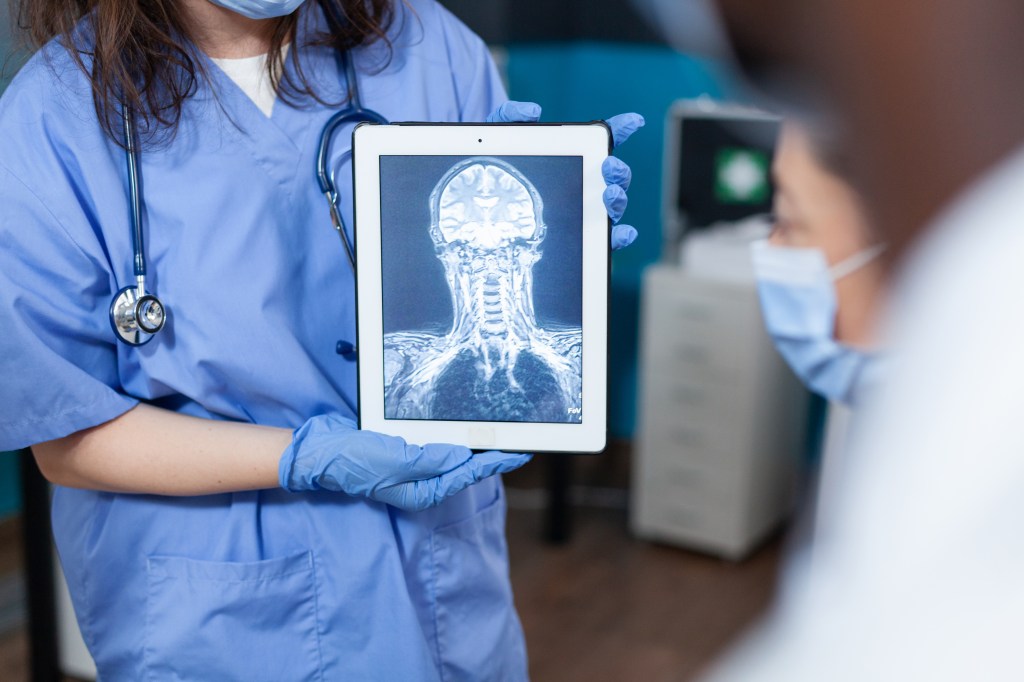

EXPERIMENTAL DESIGN
We work with clinical leads, engineers, and regulatory teams to design experiments and trials that generate robust, regulatory-ready data.
This includes validation studies for medical devices, usability assessments for digital tools, and early feasibility protocols.
Our expertise ensures that your data not only supports safety and efficacy but also tells a clear, defensible story to regulators and investors.
